Commercial winemakers who rely on grape suppliers and hobbyists are both at the mercy of the harvester, who exercises control over the most critical stage of winemaking. The concentration of various grape ingredients that will impact the taste of wine vary significantly. During ripening, the levels of sugar, phenolic compounds and aromas rise while the amount of acid decreases. A few weeks before picking grapes, results of laboratory analyses and weather forecasts are consulted to choose the optimal time for harvesting.
No winemaker in my extended family relies on concentrated grape juice from kits. Instead they get their fruit from 36 pound wooden cases called lugs. These make their way to Montreal from California by either truck or train. Upon close inspection, a few bunches don't exactly appear to be fresh off the vines. But most are impeccable and delicious, far sweeter than table grapes. As a kid I would often sneak into the garage to smell and taste them.
The two main sugars in grapes are fructose and glucose, accounting for a whopping 18 to 25 % of their content. Pectin only accounts for about 0.06%, which is why it's necessary to add pectin when making grape jam. But in winemaking that same amount of pectin has to break down; otherwise it reduces the clarity of wine. In dry wines, most of the sugar will have been converted to alcohol during fermentation, an oxygen-independent reaction that provides yeast with adenosine triphosphate (ATP).
Why is the acid-level important in winemaking? Low acidity (high pH), common in grapes that are too sweet because they were grown in excessively warm climates, lowers the amount of subtle flavors in the grapes and wine. There is also an optimum pH for fermentation because, as Pasteur demonstrated, it is an enzyme-driven process. Prior to fermentation, pH is measured either by titration or more conveniently with a pH meter, and if it is too high, an acid blend is added. The acid blend consists of the three organic acids that are found in grapes: citric, malic and tartaric, the latter is less common in fruits but the most common in grapes. It led to Pasteur's
discovery of enantiomers, molecules that are mirror images of one another. (The original Pasteur experiment, however, has been difficult to replicate.) Yeasts themselves will then add a small amount of other acids to wine.
Phenolic compounds which include the same type of compounds that make autumn leaves red affect the astringency and color of wine. Normally fermentation occurs in stainless steel tanks. But some chardonnay wines undergo fermentation in oak barrels and owe part of their taste to tannins transferred from wooden barrels.
When grapes have the right balance of sugar and acid, they are harvested and are quickly brought to the winery to be destemmed and crushed. Destemming the grapes prevents the stalks and stems from being crushed with the grapes, which would produce an excess of tannins. Red grape juice or must is fermented with their skins to extract color. White grapes are pressed after they are crushed so only the juice is kept for fermentation. A five to ten percent solution of sodium metabisulfite (Na2S2O5) is added to kill wild yeast on the grapes. This creates an equilibrium with sulfur dioxide(SO2), the truly active ingredient. The wild yeasts are too varied in composition and are often intolerant to wine alcohol concentrations, causing fermentation to stop prematurely, leaving a high residual sugar content in the finished product. In addition, SO2, inhibits enzymes that oxidize phenolic compounds responsible for discoloring wine. If a minimum of 80 ppm of SO2 is not present, more SO2 gas is bubbled into the juice, but the legal limit is 200 ppm.
Due to SO2 treatment, fermentation won't start until a selected wine yeast is reintroduced, usually a pure culture of Saccharomyces cerevisiae. Interestingly my father and brother have at times started with the same grapes, with my dad refusing to add metabisulfite and yeast to his vat. You can guess which wine usually comes out looking and tasting better.
Fermentation is an exothermic process (it releases heat). But in winemaking, the temperature cannot exceed 29oC for red wines or 18oC for white wines), otherwise the growth of yeast cells may stop. Moreover, a lower temperature is desirable because it prevents the loss of volatile aromas and flavours. In homemade wine, excess heat is usually not a problem, but since large commercial vats have lower surface to volume ratios, they cool to slowly on their own.
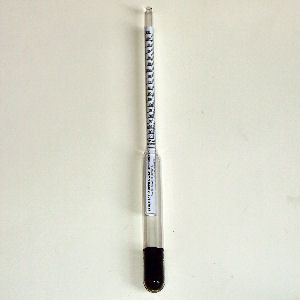
As sugars get converted to alcohol, the density of the mixture decreases. Since an aqueous alcohol is less dense than an aqueous sugar solution, a hydrometer can help determine when fermentation has stopped, usually when the specific gravity has fallen to about 1.000. An alternative to measuring sugar concentration is the Fehling reagent. I've also watched my brother use a hydrometer prior to fermentation to predict the wine's potential alcohol content.
The freely run juice after fermentation is of the best quality. Going for quantity by squeezing the pulp dry severely compromises the quality of wine. It should be used only for later distillation of alcohol, which can then be added to other products. My father is fully aware of this fact, but driving his cost down to two dollars per bottle takes priority over fine taste.
To clarify the wine, the fermented juice is transferred into a settling vat, or if made on a smaller scale, into a demijohn. In these, suspended yeast cells, cream of tartar and particles of skin and pulp settle to the bottom of the container. As the yeast cells break down within the precipitate, they stimulate the growth of Lactobacillus bacteria that convert the wine's malic acid into lactic acid. This malolactic fermentation process is especially important in wines made from highly acidic grapes because lactic acid is a weaker acid than malic acid. (Bacteria decarboxylate malic acid , thus removing one of the acidic carboxyl groups), so it mellows the wine's taste.
After the demijohn stage, the wine is repeatedly racked to leave behind less and less sediment called lees. During the repeated racking, the wine is also given a chance to rid itself of the excess carbon dioxide from fermentation. As the CO2 escapes, oxygen enters the wine with each transfer, helping eventually to age the wine.
The wine's final flavor comes from its blend of phenolics, acids and sugars. But aromatic compounds are said to give it "character". In reality we do not taste wine without also smelling it, and molecular gastronomists remind us that we don't perceive what we smell directly through the nose in the same way as what we smell through the mouth. The concentrations of volatiles is quite low: in the 1 to 4 ppm range. The fruity or floral smells are due to the monoterpenes (natural products with repeating units of carbon and hydrogen but ending with an OH group) containing citronell, alpha-terpineol, geraniol and linalool. Aged wines have compounds like vitispirane and TDN(1,1,6-trimethyl-1,2-dihydro naphtalene).
In the CBC documentary by Josh Freed, "The Trouble With Experts", a professional winemaker and university professor fooled wine-tasting experts by switching the labels on them. Non-connoisseurs, however, preferred the more expensive wines, without knowing which was which. The idea was that expectation plays a role in judgment, but there is a distinguishing chemistry between different wines, nevertheless, one that is not always worth the exuberant price difference.
Occasionally, despite my dad's outdated winemaking methods, I have caught myself saying, "You know, this wine isn't half-bad!" Especially after the first glass.
Edited by Ted R. Uva
Other Sources:
http://nzic.org.nz/ChemProcesses/food/6B.pdf
Amerine, Maynard. Wine. Scientific American. August 1964.
United States Department of Agriculture